Energy 101
In high school we learnt that energy cannot be created or destroyed. So why all this concern about using energy?
I really can’t put it better than MacKay*:
“Isn’t energy conserved? We talk about “using” energy, but doesn’t one of the laws of nature say that energy can’t be created or destroyed?
Yes, I’m being imprecise. This is really a book about entropy – a trickier thing to explain. When we “use up” one kilojoule of energy, what we’re really doing is taking one kilojoule of energy in a form that has low entropy (for example, electricity), and converting it into an exactly equal amount of energy in another form, usually one that has much higher entropy (for example, hot air or hot water). When we’ve “used” the energy, it’s still there; but we normally can’t “use” the energy over and over again, because only low entropy energy is “useful” to us. …It’s a convenient but sloppy shorthand to talk about the energy rather than the entropy,…”
(*from “Sustainable Energy – without the hot air” by David JC Mackay www.withouthotair.com – which, if I had the knowledge and skill, would be the book I would have liked to write…)
The other way to think about this idea is to think of concentrated versus distributed “energy” (and we will drop the quotation marks from here on).
So electricity in a high-voltage distribution line is concentrated, while the heat given off by an electric radiator, once it’s been convected around a room in the form of warmed air is quite diffuse. This of course makes oil and its derivatives such an ideal energy source; easy to transport, easy to store, easy to ignite, and full of concentrated energy.
We could split the discussion of alternative energy sources into those where we take diffuse energy, like sunlight and wind, and turn it into concentrated energy like electricity – which in turn enters the existing energy distribution system. Turning biomass or algae into liquid fuel is much the same, except that we have intermediate (and, in the realists opinion, wasteful) steps of turning sunlight into a complex biological entity, and then refining that back into a simple volatile hydrocarbon.
On the other side we have processes where we use relatively diffuse energy, “concentrate” it somewhat, and use the diffuse energy for diffuse purposes. In plain language; why use concentrated energy that can melt metal, like electricity or oil and gas flames to heat water, which is then pumped around a house to raise the inside temperature by a few degrees? The only reason to do that is because the fuel has to be transported to the house and electricity lines, gas pipes and oil tankers are a very convenient way of transport. If the energy can be gathered at the house directly, by solar hot water panels for instance, the warm water so generated is quite enough to heat the house to a comfortable level. After all we frail humans only have a very limited comfort zone, and water even at a paltry 180F is much too hot for us.
For a formal discussion on energy here is the Wikipedia entry.
Energy sources
The various sources of energy are covered in exhaustive detail elsewhere (in books and countless web sources), but for sake of completeness here are the major sources we can realistically use:
The SUN

– directly of course, but also in a long list of indirect ways:
- Wind, Waves and Currents

- The whole hydraulic cycle giving us dams and running streams (and hurricanes)

- Photosynthesis, giving us short term crops like corn, grass, sugar cane, algae and so forth, but also at increasingly long production times (and corresponding energy concentration): wood, peat, methane deposits, natural gas, coal and oil. Under this category we can also lump all the energy generated by animals, like draft oxen, or extracted from animals, like whale oil. The animals of course either eat plants, or eat other animals that eat plants. (see also my entry on biochar and biomass).
- Stored solar energy in air, water and soil. This is commonly called “geothermal” energy when used in conjunction with heat-pump technology. Here we will try and be careful and refer to ground-source heat-pumps rather than “geothermal”, and use the word geothermal only for the heat contained in the earth (see below).
Gravity – the influence of the moon and sun on the earth, causing tides in water and also in the earth itself , continuing to heat the earth (again see geothermal)
Geothermal – heat from the molten core of the earth, left over from the initial formation, radioactive decay of rocks and the gravitational tides in the earth structure. For completeness I suppose I need to include those strange plants and animals to live around geothermal deep ocean vents independent of photosynthesis.
Nuclear fusion – in a highly concentrated, but not very controlled, form as nuclear bombs, and in a more controlled form as nuclear reactors.
Nuclear fission – in concentrated form known and loved by all as H-bombs (See “The Road“). The research into controlled fusion has been going on as long as I remember (and I can remember several decades) and has not reached any satisfactory point. The latest entry from Wikipedia states that we have achieved a burst of power generation lasting half a second, and still below the input power needed to start the process. If we ever get through the technology barrier this may be the holy grail of cheap, infinite energy. But don’t hold your breath. In the meantime we have a free fusion generator hanging in the sky, for about half of each day. I will not mention cold fusion, except to mention it.
Sunlight is amazingly abundant
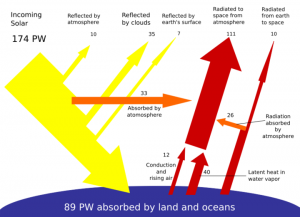
The diagram is from Wikipedia and shows the perfect balance between incoming radiation and what goes out again. Of course if the balance is even very slightly off we either get cold and lifeless, like Mars, or we get hot and lifeless, like Venus. We would need to be quite foolish to ignore any even minor chance of going in either direction.
PW in the diagram is PetaWatts or 174 * 10**15Watts falling on the total earth surface.
The sun, falling on my quarter acre lot in NJ, deposits between 1630 and 5750 KWh per day*, compared to my use of between 11 to 20 KWh per day of electricity and maybe double that of natural gas.
(*The maths: quarter acre is 1000 sqm, give a bit. Incident sunlight is between 1.63 (in December) and 5.75 (in June) KWh/sq meter/day based on Nasa figures for NJ)
Solar power as an alternative source for all electricity
A provocative paper by David Mills and Robert Morgan of Ausra outlines the possibility of generating all of the current electricity for the USA from a single facility with a footprint of 145km per side. That’s just over 8000 square miles, or a square of 90 miles per side. Granted this is a sizable chunk of land, but not so big when compared to the world’s desert areas. The down side of the proposal is the grid – how to get the electricity from “there” to where it is needed. The second problem is that the sun does not shine all day, and even in the most sunlit desert not on every day of the year. How to store the energy, in the most economic manner, for the night and the rainy day is a matter of intense competitive research.

Solar thermal array by Ausra on I5 north of Bakersfield, CA. The parabolic reflectors are at ground level hidden by the fence. Elevated boiler tubes are visible behind the power poles. Apologies for the poor quality of the photo taken while driving on I5 in June '09
Units of measurement
Mackay: “SI stands for Syst`eme Internationale. SI units are the ones that all engineers should use, to avoid losing spacecraft. (admin note: MacKay presumably refers to this story: On September 23, 1999 NASA lost the $125 million Mars Climate Orbiter spacecraft after a 286-day journey to Mars. Miscalculations due to the use of English units instead of metric units apparently sent the craft slowly off course.)
SI units:
- energy one joule 1 J
- power one watt 1W
- force one newton 1N
- length one metre 1m
- time one second 1 s
- temperature one kelvin 1K”
We will not repeat all the definitions, tables and conversion factors here. They can be found in MacKay’s book, and on many web sites. Life would be so much easier if we could just convert everything to KWh instead of using “annoying” units.
Just for fun here are some conversions and size indicators:
Work and Energy use the same units – the SI joule
foot-pound =1.3558 J
BTU (or BThU if you prefer) =1055J, or the energy in a flaring match head (very precise that…)
hp-hour = 2.6845MJ
KWh = 3.6*10**6J or 3.6 megajoules
Therm = 100,000 BTU or approx. 100 cubic feet of natural gas – but of course nobody can agree on that either – A European Therm is 105,506,000J, a US Therm is 105,480,400 and the British have to have it at 105,505,585.257348J
Then the Europeans have a “thermie”, which raises a metric tonne of water by 1 degree C.
Some so-called “units” are just a rule of thumb short-hand and we will expand on these a little more.
In Germany there is much talk of the “one liter house”. What it means is an energy efficient house that can be heated for one year with one liter of oil per square meter. Translated to the US that means 1 gallon per 43 sq ft per year. With an average of US house house size of over 2000 sqft, that works out at about 50 gallons of oil, or $150 per year! (Everything approx. and based on 1 liter of oil having an energy equivalent of 10KWh) Very nice compared to the usual heating costs in the North East.
Specific trades have their own units to add to the confusion – e.g.: “Air conditioner equipment power in the U.S. is often described in terms of “tons of refrigeration.” A “ton of refrigeration” is defined as the cooling power of one short ton (2000 pounds or 907 kilograms) of ice melting in a 24-hour period. This is equal to 12,000 BTU per hour, or 3517 watts. Residential central air systems are usually from 1 to 5 tons (3 to 20 kilowatts (kW)) in capacity” (from Wikipedia on air conditioning)
And a bit more on joules and KWh:
A human adult “uses” 1500-2000 Calories (which are really Kilogram calories), or about 6 to 8 MJ, say 2 KWh. – running a toaster for an hour and a half…
What about those magic R and U values for insulation? Different between Europe and the US of course; 1R (US) = 0.1761R (SI), or 13R (US), equivalent to about 4 inches of fibreglass batt, = 2.3R (SI). 1U = 1/R(SI). So R13 is 0.44U. Easy…
A “red herring” on entropy: Diffuse energy is not much good to anybody, that’s why all this effort to either find it in concentrated form (gas shales, ANWR drilling etc., or to concentrate it by some smart means (heat pumps). An analogy is all the gold in the earth’s oceans, about a kilogram for each of the 6 billion of us – something over $30,000 at today’s prices. (The math? Gold occurs in seawater at a concentration of 6 parts/trillion = 6Kg per cubic kilometer of ocean, some 1.3billion cubic km in all the oceans…). But all of it not much good to anyone in it’s distributed, very high entropy nature.

[…] and then turning that highly concentrated (low entropy) energy into warm air or water. See Energy 101 for a slightly more detailed discussion and […]